How do we create polymers
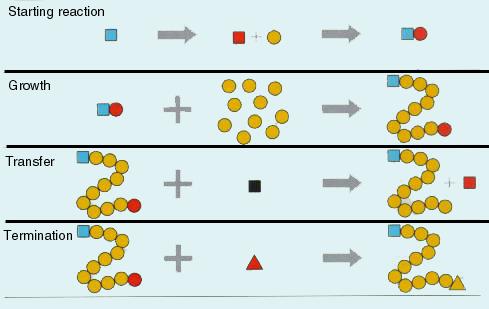
- a reaction (polymerisation)
- condensation (poly-condensation)
- or addition (poly-addition).
Depending on the momomers that particpate in the process the chain may grow into a linear assembly or more complex topologies. For us the
- topology and the
- inner structure
will be the two important factors that determine the chemical and the physical properties of the polymer material.
Examples of linear chain materials are nylon and terylen thermo-platic material). To start the condensation reaction one needs "starters". The degree of polymerisation, i.e., the number of monomers in the chain, can be controlled through the participating reaction elements.
